Abstract
Introduction: Despite achieving a deep hematologic response after therapy, systemic light-chain amyloidosis (AL) patients may not attain an organ response, possibly due to a persistent plasma cell clone below the detection threshold of traditional serological methods. Previous studies demonstrated the utility of minimal residual disease (MRD) by mass spectrometry (Dispenzieri et al. Blood Cancer J. 2020) and next-generation flow cytometry (Palladini et al. Blood Cancer J. 2021) in AL. MRD by next-generation sequencing (NGS) was studied prospectively in AL (Sarosiek et al. Blood Cancer J. 2021), however, there is no data of using NGS in the real-world setting in AL. This study aims to assess the feasibility of NGS in AL patients in the real-world setting.
Methods: We performed a single-center retrospective study of AL patients, who underwent clone identification (ID) using NGS on bone marrow aspirates at the University of California San Francisco between September 2016 and December 2021. All AL patients must have had biopsy-proven systemic AL and amyloid typing performed at diagnosis. Patients who developed AL from an underlying lymphoma were excluded.
Marrow samples were sent to Adaptive Biotechnologies Inc. (Seattle, WA) for initial clonal ID using the clonoSEQ® Assay. Patients with a trackable clone on initial ID sample had subsequent marrow specimens sent for MRD testing.
Cardiac and renal staging were determined by established criteria (Palladini et al. Blood 2015; Palladini et al. Blood 2014) at diagnosis. At the time of MRD measurement, hematologic responses were graded using previously published criteria (Kastritis et al. NEJM 2021).
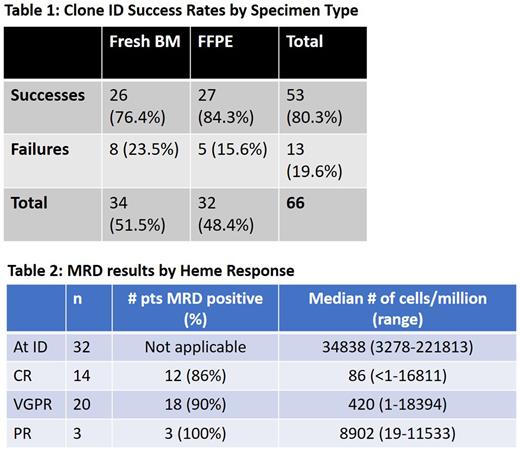
Results: We identified 66 patients who had bone marrow samples sent for NGS clone ID. Of these 66 patients, 53 (80.3%) returned with a successfully identifiable clone while 13 patients (19.6%) did not due to either polyclonality (11 patients) or quality control issues (2 patients). At least 1 MRD tracking measurement was available in 32/53 (60.3%) of these patients.
Of the successful IDs, the percentage success rate was similar in patients who had their samples extracted from fresh bone marrow (26/53, 76.4%) compared to patients who had their samples extracted from formalin-fixed paraffin-embedded (FFPE) tissue (27/53, 84.3%) (Table 1). Median bone marrow plasma cell % for successful and failed ID was 10% (range 5-52%) and 10% (range 3-90%) respectively.
Of the ID successful patients, 32 patients had ≥1 MRD performed. Median age was 67 years (range 47-87) and 25% were female, 68% lambda AL. Median iFLC, dFLC and BMPC were 207.3 mg/L (range 19-4422.6), 199.1 mg/L (range 2.4-4415.5) and 10% (range 5-80) respectively. Cardiac involvement occurred in 28 (87%) patients with cardiac stage 1/2/3A/3B in 7/13/4/3 patients. Renal involvement occurred in 22 (68%) patients with renal stage 1/2/3 in 6/11/5 patients. Patients received a median of 2 lines of therapy (range 1-8), with 12 (37.5%), 26 (81.3%) and 8 (25%) patients having underwent an autologous stem cell transplant, exposure to daratumumab and exposure to venetoclax respectively.
The number of patients who had at least 1, 2, 3, ≥4 MRD measurements were 32 (100%), 18 (52%), 10 (31%) and 5 (15%) respectively. Of the 14 patients with CR when MRD was measured, 12 (86%) were MRD positive, with median of 86 cells/mil (range <1-16811) (Table 2). Similarly, among 20 patients who were in VGPR, 18 (90%) had MRD positivity with median of 420 cells/mil (range 1-18394).
Conclusion: Clone ID by NGS on bone marrow samples from AL patients is feasible in a real-world setting, regardless of fresh versus FFPE specimens. Although the median # of cells/million differed by depth of hematologic response, there was much overlap in the range as well as % patients with MRD positivity between response categories. Organ outcome analyses are ongoing and will be updated at time of presentation. Further studies with a larger number of AL patients are needed to determine the utility of MRD by NGS on patient outcomes.
Disclosures
Chung:Merck: Research Funding; Caelum: Research Funding; AbbVie: Research Funding; BMS/Celgene: Research Funding; Sanofi: Honoraria; Janssen: Research Funding; Cellectis: Research Funding. Shah:AstraZeneca: Current Employment, Current equity holder in publicly-traded company; Celgene/BMS, Janssen, Bluebird Bio, Sutro Biopharma, Teneobio, Poseida, Nektar, Precision Biosciences: Research Funding; Aztra Zeneca: Current Employment, Other: stock ownership; GSK, Amgen, Indapta Therapeutics, Sanofi, CareDx, Kite, Karyopharm, Oncopeptides,: Consultancy. Wolf:Adaptive Biotechnologies: Consultancy. Martin:GlaxoSmithKline and Legend Biotech: Consultancy; Amgen, Johnson & Johnson / Janssen, Sanofi, and Seattle Genetics: Research Funding; Legend Biotech: Consultancy. Wong:Catalent Biologics: Consultancy; Bristol Myers Squibb: Research Funding; Caelum: Research Funding; Genentech: Research Funding; Fortis: Research Funding; GSK: Research Funding; Janssen: Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees; Dren Bioscience: Consultancy; Patient Discovery: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal